FDA Cuts Drug Review Time To Weeks For Companies Promoting 'National Priorities,' Reminiscent Of Covid Era "Operation Warp Speed"
"During the COVID-19 pandemic, review processes that took a year were performed in weeks. We believe this is a clear demonstration that rapid or instant reviews are possible."
Earlier this week, the Food and Drug Administration (FDA) announced that it would provide vouchers to pharmaceutical and biotech companies to fast track the approval of new drugs and vaccines within “weeks,” a process reminiscent of President Donald Trump’s “Operation Warp Speed” in 2020, where drug companies were no longer required to undergo multiple rounds of clinical testing for their vaccines and therapeutics, which would normally take at least several years to fully perform.
The FDA’s review time will now be truncated to at least a year to now a matter of a couple of months if not weeks.
The policy shift was announced on June 17th in a headline press release on the FDA’s website, where the FDA will provide “limited vouchers” to companies aligned with “U.S. national priorities.”
The press release states (emphasis mine):
The U.S. Food and Drug Administration today announced its Commissioner’s National Priority Voucher (CNPV) program to enhance the health interests of Americans. The new voucher may be redeemed by drug developers to participate in a novel priority program by the FDA that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor’s final drug application submission.
The new CNPV process convenes experts from FDA offices for a team-based review rather than using the standard review system of a drug application being sent to numerous FDA offices. Clinical information will be reviewed by a multidisciplinary team of physicians and scientists who will pre-review the submitted information and convene for a 1-day “tumor board style” meeting.
The FDA plans in the first year of the program to give a limited number of vouchers to companies aligned with U.S. national priorities. In addition to receiving the benefits of this program, the agency may also grant an accelerated approval, if the product for which the voucher is used meets the applicable legal requirements for accelerated approval. The new review program will also include enhanced communication with the sponsor throughout the process. The FDA Commissioner will use specific criteria to make the vouchers available to companies that are aligned with the national health priorities of:
Addressing a health crisis in the U.S.
Delivering more innovative cures for the American people.
Addressing unmet public health needs.
Increasing domestic drug manufacturing as a national security issue.
To qualify, sponsors must submit the chemistry, manufacturing, and controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. Sponsors must also be available for ongoing communication with prompt responses to FDA inquiries during the CNPV review. The FDA reserves the right to extend the review window if the data or application components submitted are insufficient or incomplete, if the results of pivotal trial(s) are ambiguous, or if the review is particularly complex.
Vouchers can be directed by the FDA towards a specific investigational new drug of a company or be granted to a company as an undesignated voucher, allowing a company to use the voucher for a new drug at the company’s discretion and consistent with the program’s objectives.
FDA Commissioner Marty Makary M.D., M.P.H., said in a statement:
“Using a common-sense approach, the national priority review program will allow companies to submit the lion’s share of the drug application before a clinical trial is complete so that we can reduce inefficiencies. The ultimate goal is to bring more cures and meaningful treatments to the American public.
“As a surgical oncologist, we often made multidisciplinary decisions with a team of doctors on major life-and-death questions for patients, incorporating the latest medical studies in a 1-day tumor board-style discussion. This voucher harnesses that model to deliver timely decisions for drug developers.”
Principal Deputy Commissioner Sara Brenner, M.D., M.P.H., added:
“This approach capitalizes on frequent communication with sponsors, which can be a powerful tool in reducing wasted time. We are confident this more efficient process can be achieved without cutting any corners on safety or scientific evaluation.”
Before the FDA change was formally announced, Commissioner Makary published a piece in The Journal of the American Medical Association (JAMA) titled “Priorities for a New FDA,” promoting accelerated approval for drugs and treatments in the U.S. He wrote:
Why does it take more than 10 years for a new drug to come to market? Why are childhood chronic diseases so prevalent? And how can regulators adapt to meet the challenges facing clinicians today? These questions are at the forefront for the US Food and Drug Administration (FDA).
The US leads the world in sophisticated cell and gene therapies and other innovative treatments, but in terms of the health of the population, our medical system has been a 50-year failure. Forty percent of US children now have a chronic medical condition and 1 in 6 has a neurodevelopmental disorder. Life expectancy has plateaued or fallen and is not commensurate with health care spending.
Moreover, Makary and senior FDA official Dr. Vinay Prasad added in the JAMA paper:
“Here are our priorities for a new FDA. During the COVID-19 pandemic, review processes that took a year were performed in weeks. We believe this is a clear demonstration that rapid or instant reviews are possible.
“The time from when pivotal trial results are known to when decisions are made must be shortened. This has implications for public welfare and will improve the risk-reward calculation of drug development.”
The Associated Press noted that this change reflects a “truncated process used to authorize the first COVID-19 vaccines under Operation Warp Speed.”
On top of this, the AP revealed that the FDA is potentially looking to ‘fast track’ for certain drug requirements and randomized studies. The AP added:
Separate from this week's announcement, Makary recently suggested the FDA should be willing to ease its scientific requirements for certain drugs targeting rare conditions. In such cases, the agency could consider waiving its requirement for randomized studies, in which researchers track patients over time to evaluate drug safety and effectiveness. Such trials are generally considered the gold standard of medical research, though the FDA has increasingly been willing to accept smaller, less-definitive studies for rare or life-threatening diseases.
In several recent cases, the FDA has faced criticism for approving drugs based on preliminary data that didn't ultimately show benefits for patients.
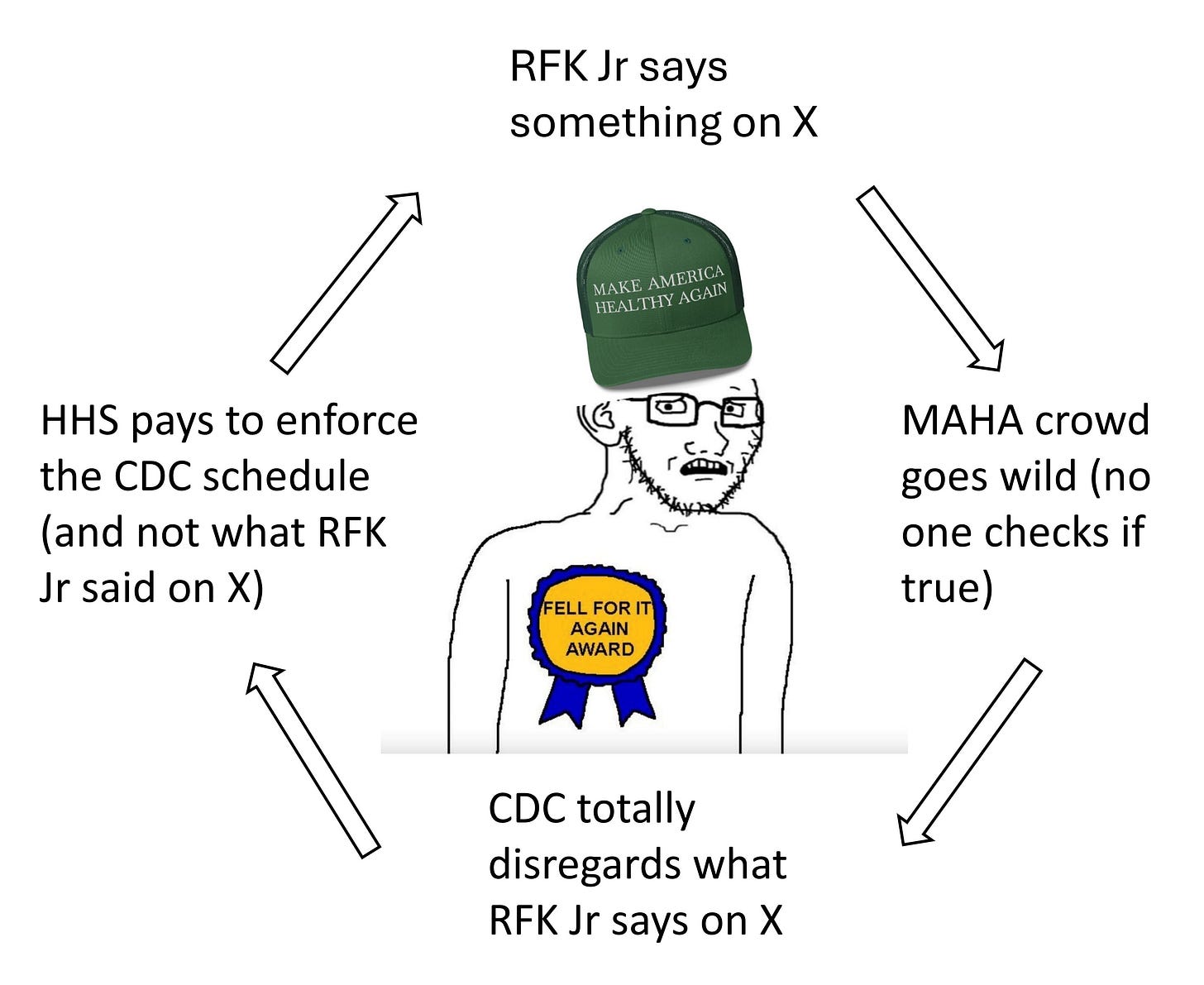
The push to rapidly accelerated drug approvals is the opposite approach that Makary and his boss, Health Secretary Robert F. Kennedy Jr., have taken on vaccines.
Promising a “return to gold-standard science,” Kennedy previously announced that all new vaccines would have to be compared to placebo, or a dummy shot, to win approval. Kennedy and Makary also have announced a stricter policy on seasonal updates to COVID-19 shots, saying they will have to undergo new testing before they can be approved for use in healthy children and most adults.
Dr. Markary was one of the authors of the Make America Healthy Again “MAHA Report,” commissioned by Health and Human Services (HHS) Secretary Robert F. Kennedy Jr.
The MAHA authors listed ten points they wish to see implemented:
Addressing the Replication Crisis: NIH should launch a coordinated initiative to confront the replication crisis, investing in reproducibility efforts to improve trust and reliability in basic science and interventions for childhood chronic disease.
Post-Marketing Surveillance: NIH and FDA should build systems for real-world safety monitoring of pediatric drugs and create programs to independently replicate findings from industry-funded studies.
Real-World Data Platform: Expand the NIH-CMS autism data initiative into a broader, secure system linking claims, EHRs, and environmental inputs to study childhood chronic diseases.
AI-Powered Surveillance: Create a task force to apply AI and machine learning to federal health and nutrition datasets for early detection of harmful exposures and childhood chronic disease trends.
GRAS Oversight Reform: Fund independent studies evaluating the health impact of selfaffirmed GRAS food ingredients, prioritizing risks to children and informing transparent FDA rulemaking.
Nutrition Trials: NIH should fund long-term trials comparing whole-food, reduced-carb, and low-UPF diets in children to assess effects on obesity and insulin resistance.
Large-scale Lifestyle Interventions: Launch a coordinated national lifestyle-medicine initiative that embeds real-world randomized trials—covering integrated interventions in movement, diet, light exposure, and sleep timing—within existing cohorts and EHR networks.
Drug Safety Research: Support studies on long-term neurodevelopmental and metabolic outcomes of commonly prescribed pediatric drugs, emphasizing real-world settings and meaningful endpoints.
Alternative Testing Models: Invest in New Approach Methodologies (NAMs), such as organon-a-chip, microphysiological systems, and computational biology, to complement animal testing with more predictive human-relevant models.
Precision Toxicology: Launch a national initiative to map gene–environment interactions affecting childhood disease risk, especially for pollutants, endocrine disruptors, and pharmaceuticals.
In the subsection Growth of the Childhood Vaccine Schedule, the report says,
“Vaccines benefit children by protecting them from infectious diseases. But, as with any medicine, vaccines can have side effects that must be balanced against their benefits. Parents should be fully informed of the benefits and risks of vaccines. Many of them have concerns about the appropriate use of vaccines and their possible role in the growing childhood chronic disease crisis.
“Our understanding of vaccine safety and any links to chronic disease would benefit from more rigorous clinical trial designs, including the use of true placebos, larger sample sizes, and longer follow-up periods. Many vaccines on the CDC’s childhood schedule involved small participant groups, had no inert placebo-controlled trials, and had limited safety monitoring, some lasting six months or less—raising concerns about the ability to detect rare or long-term adverse effects.”
Interestingly enough, the MAHA Report notes that the majority of the FDA is controlled by big-pharma execs. “9 of 10 past FDA commissioners have gone on to work in the pharmaceutical industry; similarly, roughly 70% of FDA medical examiners ultimately find employment in the industry,” the report says.
At the end of May, Moderna announced in a press release the approval by the FDA for its COVID-19 Vaccine mNEXSPIKE (mRNA-1283), “for use in all adults 65 and older, as well as individuals aged 12-64 years with at least one or more underlying risk factor as defined by the Centers for Disease Control and Prevention (CDC),” the press release explained, The WinePress reported.
AUTHOR COMMENTARY
I am going to keep posting this meme until the cows come home:
Job 13:4 But ye are forgers of lies, ye are all physicians of no value.
This is a GIFT to big-pharma and the biotech killers. We all saw and bore witness to what has and is happening to those who voluntarily accepted the Covid-19 mRNA death shots that were approved via “warp speed” methods under Trump, the same operation and vaccines he continues to praise to this day, and refuses to ever acknowledge the mass harm that they have caused. But this is not surprising considering that I reported blatant evidence that proves Trump and his first administration knew about the vaccines because he approved contracts for their production before he even announced Covid to Americans in mid-January, on top of an executive order he signed in September 2019 to create new flu vaccines.
So now moving forward, all drugs and vaccines are going to get that ‘golden’ MAHA and MAHA rubber stamp with next to no testing and years-long clinical trials, in a room full of the same old big-pharma execs, salivating and rubbing their mitts together at all the money they are going to bring in. If big-pharma wants a drug fast, they’ll get it without impunity because Bobby told us they’re safe. But hey, we got that Red 40 out of your cookies and Steak’n Shake fries will be cooked in beef tallow! Winning!
But we should not be surprised by this. Bobby was never “anti-vax”: that is a mainstream lie that RFK Jr. has for years refuted; but the so-called “Health Freedom Movement” of MAGA idolaters and New Agers and government spooks don’t want you to know this.
Before assuming the head role at HHS, Pfizer CEO Albert Bourla met with RFK Jr.. Bourla said during an earnings call:
“I don’t want to speak about the details of what we discussed during that dinner because I want to respect the privacy, but we developed a good relation with Mr. Kennedy. If he’s confirmed, we will work with him to make sure that we advance the right policies.
“The President is extremely proud, and of course, we are extremely proud, that [Pfizer] basically delivered a vaccine through this landmark golden standard program, the Operation Warp Speed […] that saved millions of lives.”
Read that report to understand just how connected all of this is. New cape, same villain.
So, when we inevitably get this “bird flu pandemic,” get ready to have a flood of mRNA shots and drugs flood the markets at “warp speed;” to be injected into our livestock and pets, and into people, and watch more people and animals keel over in the process.
Ecclesiastes 10:19 A feast is made for laughter, and wine maketh merry: but money answereth all things.
See my other reports on the colossal embarrassment and lies of MAHA and Kennedy:
US And Argentina Announce Partnership To Create Alternative To World Health Organization
The Lord Of Glory: The Detailed Guide To Who God Is – Available Now!
·On one of his missionary journeys, the apostle Paul visited Athens, Greece, where he said he witnessed “the city wholly given to idolatry,” and who were “too superstitious” and worshipped a plurality of gods and deities, though the people acknowledged that there was still one God above all that was a mystery to them. When questioned by the philosophers …
[7] Who goeth a warfare any time at his own charges? who planteth a vineyard, and eateth not of the fruit thereof? or who feedeth a flock, and eateth not of the milk of the flock? [8] Say I these things as a man? or saith not the law the same also? [9] For it is written in the law of Moses, Thou shalt not muzzle the mouth of the ox that treadeth out the corn. Doth God take care for oxen? [10] Or saith he it altogether for our sakes? For our sakes, no doubt, this is written: that he that ploweth should plow in hope; and that he that thresheth in hope should be partaker of his hope. (1 Corinthians 9:7-10).
The WinePress needs your support! If God has laid it on your heart to want to contribute, please prayerfully consider donating to this ministry. If you cannot gift a monetary donation, then please donate your fervent prayers to keep this ministry going! Thank you and may God bless you.










So very much could be said, however I'll be brief. "The FDA’s review time will now be truncated to at least a year to now a matter of a couple of months if not weeks."<----Basically, what they are saying is . . . the time frame of the past can no longer be utilized because no one would survive the trials. Therefore, if we say it is safe, the plebs will accept more bioweapons leading to debilitating health at best, if not death.
It also fits in with this: "Pfizer CEO Albert Bourla met with RFK Jr.. Bourla said during an earnings call:
“I don’t want to speak about the details of what we discussed during that dinner because I want to respect the privacy, but we developed a good relation with Mr. Kennedy. If he’s confirmed, we will work with him to make sure that we advance the right policies."<---The policy similar to the c-19 protocol? Yeah, I bet so!
"- Addressing the Replication Crisis: NIH should launch a coordinated initiative to confront the replication crisis, investing in reproducibility efforts to improve trust and reliability in basic science and interventions for childhood chronic disease. [translation: "reproducibility efforts" = if you all use the same chatbot, you'll get the same results]
- Post-Marketing Surveillance: NIH and FDA should build systems for real-world safety monitoring of pediatric drugs and create programs to independently replicate findings from industry-funded studies.[translation: no more trials, you are the guinea pigs. see also: "post-marketing surveillance"]
- Real-World Data Platform: Expand the NIH-CMS autism data initiative into a broader, secure system linking claims, EHRs, and environmental inputs to study childhood chronic diseases. [translation: everything your kid does goes into the cloud and becomes a social credit score]
- AI-Powered Surveillance: Create a task force to apply AI and machine learning to federal health and nutrition datasets for early detection of harmful exposures and childhood chronic disease trends. [translation: our computer says you have possible dioxin exposure from your gas grill! everybody out of the neighborhood and into the 15 minute prison!]
- GRAS Oversight Reform: Fund independent studies evaluating the health impact of selfaffirmed GRAS food ingredients, prioritizing risks to children and informing transparent FDA rulemaking.
[translation: you vill eat zee bugs]
- Nutrition Trials: NIH should fund long-term trials comparing whole-food, reduced-carb, and low-UPF diets in children to assess effects on obesity and insulin resistance.[translation: nobody gets fat eating zee bugs]
- Large-scale Lifestyle Interventions: Launch a coordinated national lifestyle-medicine initiative that embeds real-world randomized trials—covering integrated interventions in movement, diet, light exposure, and sleep timing—within existing cohorts and EHR networks.[translation: lights out at 8, up at 6, an hour a week in the exercise yard with all the other inmates]
- Drug Safety Research: Support studies on long-term neurodevelopmental and metabolic outcomes of commonly prescribed pediatric drugs, emphasizing real-world settings and meaningful endpoints. [translation: your kids vill take zee tracking chip]
- Alternative Testing Models: Invest in New Approach Methodologies (NAMs), such as organon-a-chip, microphysiological systems, and computational biology, to complement animal testing with more predictive human-relevant models.[translation: because we can't test on animals since UN2030 SDGs says animals are worth more than people]
- Precision Toxicology: Launch a national initiative to map gene–environment interactions affecting childhood disease risk, especially for pollutants, endocrine disruptors, and pharmaceuticals. [translation: our computer says you have to shut down farms and factories and get on a UBI and CBDC! everybody out of the neighborhood and into the 15 minute prison!]